
Cellulose
Cellulose or tissue (from lat. cellula — "cell") are substances also directly related to sugars. Their molecules are linked with hydrogen bonds (weak interaction) and are formed from a set of (2000 to 3000) of residues of B-glucose. Cellulose is the main component of any plant cells. It is contained in the wood, the shells of some fruits (for example, sunflower seeds). Pure cellulose is a white powder, not soluble in water and not forming a paste. To estimate the "touch" pure cellulose you can take, for example, cotton wool or white poplar fluff. It's practically the same thing.
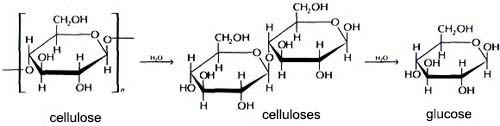
If you compare cellulose and starch, the starch hydrolysis better. Hydrolysis of cellulose is carried out in acidic solution, it first disaccharide of cellobiose forms, and then glucose.
Cellulose is widely used in industry, clean it, made a cellophane (polyethylene and the cellophane differ from each other with touch (cellophane does not seem "fat" and "rustling" then deformation), and artificial fibre viscose (lat. viscosus — "viscous").
In our body, disaccharides (e.g., sucrose, lactose) and polysaccharides (starch) with the action of special enzymes are hydrolyzed to form glucose and fructose. Such transformation can be easily produced in your mouth. If you long time chew bread, then with the action of the enzyme amylase, that contained in bread, the starch hydrolyses to glucose. When you feel a sweet taste.
Below is a schematic the hydrolysis of cellulose
Getting paper

Getting paper
What do You think, what is included in composition of the paper?! Really, is a material has a very finely twisted fibers of cellulose. Some of these fibers are united by a hydrogen bond (a bond formed between the groups - OH – hydroxyl group).
The method of producing paper was already known in ancient China in the 2nd century of our era. At that time paper was made from bamboo or cotton. Later in the 9th century of our era, this secret came to Europe.
For getting paper in the middle ages, used linen or cotton cloth. But only in the 18th century found the most convenient way of getting paper –from wood. But this paper, that we now use, began to produce only in the 19th century.
The main raw material for getting paper is a cellulose. Dry wood contains about 40% of this cellulose. The rest of the tree is of different polymers, composed of sugars of various kinds, including fructose, complex molecules, various tannins, salts of magnesium, sodium and potassium, essential oils.
Cellulose getting
Cellulose getting is associated with the mechanical processing with wood and then carrying out chemical reactions with sawdust
Coniferous trees are ground to fine sawdust. The sawdust are placed in a boiling solution containing NaHSO4 (sodium hydrosulfide) and SO2 (sulfur dioxide). Boiling is carried out at high pressure (0.5 MPa) and for a long time (about 12 hours). In the solution is a chemical reaction and results is substance hemicellulose and substance lignin (lignin is a substance that is a mixture of aromatic hydrocarbons or aromatic part of the tree) and the main reaction product – cellulose, which is drop out as sediment in the tank, where a chemical reaction go. In addition, the lignin interacts with sulfur dioxide in the solution, and resulting a ethyl alcohol, vanilla, various tannins, and yeast food.
Further, the process of cellulose getting is associated with the grinding sludge by means of rolls, resulting in is cellulose particles of about 1 mm. And when these particles enter the water, then immediately swell and form paper. At this stage the paper is still not like paper and looks like a suspension of cellulose fibers in water.
In the next phase to the paper gives its main properties: density, colour, strength, porosity, smoothness, in a container add clay, titanium oxide, barium oxide, chalk, talc and additional substances that bind the cellulose fibers.
In the next phase cellulose fibres is treated with a special dlue based on resin and rosin. It is composed of resinate. If you add in the glue potassium alum, is a chemical reaction and get aluminum resinates. This substance is able to encrust the cellulose fibers, that makes them water resistance and durability.
The resulting mass is applied uniformly on a moving sieve, where it is squeezed out and dries. Here the formation of cloth paper. To impart the paper greater smoothness and shine it passed first between a metal and then between the dense paper rolls (calendering), after that the paper is cut into sheets with special scissors.
Do you think why with the time the paper turns yellow!? It turns because that the molecules of cellulose that have been got from the tree, consist of a large number of structural units of type C6H10O5, thet with the action of ions of the hydrogen atom within a certain time lose link between each other and happens violation of the structure all chain. In this process, the paper becomes brittle and loses its original color. Still happens a acidification of paper.
In order to restore crumbling paper, apply calcium hydrogen carbonate Ca(HCO3)2), that allows to temporarily reduce the acidity. There is another, a more progressive method associated with the use of the substance diethylzinc of Zn(C2H5)2. But this substance may spontaneously ignite in air and even near of the water!
Use of cellulose
In addition, cellulose used for getting paper, use still very useful property of esterification with various inorganic and organic acids. During these reactions form esters, these are ussed in industry. Links of the fragments of molecules cellulose in it chemical reaction, not torn, and the result is a new molecule of ether group -COOR-. One of the important products of the reaction is cellulose acetate, that is formed with the interaction of acetic acid (or its derivatives, for example, acetic aldehyde) and cellulose. Is a chemical substance widely used for the manufacture of synthetic fibers such as acetate fiber.
Another useful product - trinitrocellulose. It is formed when is the nitration of cellulose with mixture of acids: concentrated sulfuric and nitric. The trinitrocellulose is widely used in the manufacture of smokeless powder (guncotton). There are cellulose dinitrate, which is used for manufacturing some types of plastics and organic glasses.