Alcohols
Properties of alcohols
Getting the alcohols
 |
AlcoholsProperties of alcoholsGetting the alcohols |
 |
| Monatomic alcohols | Polyatomic alcohols | Properties of alcohols | Getting the alcohols |
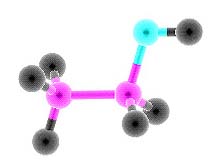
 Ethyl alcohol
 ethylene glycol |
Alcohols - derivatives of hydrocarbons, in which molecules have one or multiple hydroxyl groups OH All alcohols are divided into monatomic and polyatomic. Monatomic alcoholsMonatomic alcohols - alcohols that have one hydroxyl group.There are primary, secondary and tertiary alcohols: -the primary alcohols have a hydroxyl group near the first carbon atom, the secondary - near the second, etc. Properties of alcohols, which are isomeric, in many similar, but in some reactions they have differents. Comparing the relative molecular mass alcohols (Mr) with relative atomic masses of hydrocarbons, it is to notice that the alcohols have a higher boiling point. This is due to the hydrogen bonding between the H atom in the group OH of one molecule and the O atom in the group OH other molecules. Upon dissolution of the alcohol in the water the hydrogen bonds are formed between molecules of alcohol and water. This explains the decrease of the volume of the solution (it will always be less than the sum of the volumes of water and alcohol separately). The most prominent representative of the chemical compounds of this class is ethanol. Its chemical formula is C2H5-OH. Concentrated ethanol (wine alcohol or ethanol) is prepared from dilute solutions by distillation. It is intoxicating, and in large dose is a poison which destroys living tissue the liver and brain. It should be noted that ethanol is useful as a solvent, a preservative, a means of lowering the freezing point of any drug. Another equally well-known representative of this class is methyl alcohol (also called wood alcohol or methanol). In contrast to ethanol methanol is deadly even in small doses! First, it causes blindness, then just "kills"! Polyatomic alcoholsPolyatomic alcohols - alcohols that have multiple hydroxyl groups OH.Diatomic alcohols are called alcoholscontaining two hydroxyl group (OH group); alcohols containing three hydroxyl groups - triatomic alcohols. In their molecules two or three hydroxyl groups never be attached to the same (one) carbon atom. Dihydric alcohols also called glycol because they have a sweet taste, is characteristic of all polyatomic alcohols Polyatomic alcohols with a small number of carbon atoms are a viscous liquids, high alcohols - solid substance. Polyatomic alcohols can be getting by the same synthetic methods as saturated polyatomic alcohols.
1. Getting ethyl alcohol (wine alcohol) by fermentation of carbohydrates: The essence of the fermentation is that one of the simplest sugars - glucose received in the technique from starch with the influence of the yeast breaks down into ethyl alcohol and carbon dioxide. It is established that the fermentation process are not themselves microorganisms, but substances secreted by them - simaz. To getting ethyl alcohol is usually used vegetable raw materials rich in starch: potatoes, wheat grain, rice, etc..
2. Hydration of ethylene with the presence of sulfuric or phosphoric acid
3. The reaction of halogenoalkanes with alkali:
2) Reaction with alkali metals
3) Reaction with the halogen-hydrogen
4) Intramolecular dehydration (with the presence of a catalyst H2SO4)
5) Reaction with carboxylic acids:
6)Oxidation of alcohols.
As for polyatomic alcohols, they have a sweet taste, but some of them are poisonous. Properties of polyatomic alcohols similar to monoatomic alcohols, with the difference that the reaction is not one-to-hydroxyl group, but several at once. Ethylene glycolEthylene glycol - a typical representative of the polyatomic alcohols. Its chemical formula CH2OH - CH2OH. - dihydric alcohol. It is a sweet liquid that are able to dissolve in water in any proportions. In chemical reactions can be employed as a hydroxyl group (-OH), and two at the same time.Ethylene glycol - its solutions are widely used as anti-icing agent (chemicals). Ethylene glycol freezes at a temperature of -340C and in the cold season can replace water, for example for cooling the cars. With all the benefits of ethylene glycol you need to consider, it is a very strong poison! GlycerinWe've all seen the glycerin. It is sold in pharmacies in the dark bubbles and is a viscous, colorless liquid, sweet taste. Glycerin is a trivalent alcohol. It is very well soluble in water, boils at a temperature 220 0C.Chemical properties of glycerin are similar the properties of monoatomic alcohols, but glycerin can react with hydroxides of metals (with copper hydroxide Cu(OH)2), with the formation of glycerate metals are chemical compounds like a salts. Reaction with copper hydroxide - the typical for glycerin. In the chemical reaction forms a bright blue solution of copper glycerate EmulsifiersEmulsifiers is a higher alcohols, esters and other complex chemicals that, when mixed with other substances, such as fat, formed stable emulsions. By the way, all cosmetic products are also emulsions! As emulsifiers are often used substances, which are artificial wax (pental, sorbifolia), and triethanolamine, lecithin.SolventsSolvents are substances used for the preparation of varnishes for hair and nails. They are presented in a narrow range, as most of these substances are can ignite and harmful to the human body. The most common representative of the solvent is acetone, and amyl acetate, butyl acetate, isobutyl.There are also substances called diluents. They are usually used together with solvents for the preparation of various varnishes. |
Chemical reaction. Types of chemical reactions Chemistry tasks. Solve simple! Examples Protein. Properties of proteins Solid grease.liquid grease. Grease properties Ammonia. Properties of ammonia Bases. Properties of bases. Alkali Organic acids. Properties of carboxylic acids Potassium permanganate. Peroxide
|