Pic.1 Glucose forms a complex compound with copper salt
Pic.2 When heated with glucose formed copper hydroxide (1-valent)
Pic.3 Recovery of copper to its oxide
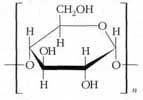
Starch
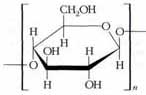 Cellulose
Cellulose


Each molecule of monosaccharide contains several hydroxyl groups (group-OH) and one carbonyl group (- C-O-H). Many monosaccharides is very difficult to distinguish from the solution in the form of crystals, as they form viscous solutions (syrups), consisting of various isomeric forms.
The most famous monosaccharide — grape sugar, or glucose (from GK. "glykys" — "sweet"), ÑáÍ12Îá.
Glucose
Glucose belongs to the class of aldehydebase — compounds containing hydroxyl and aldehyde groups. There are five hydroxyl groups and one aldehyde in a molecule of glucose. The presence of these groups in glucose can be proved using the reaction "silver mirror".
The formula of glucose usually used of shot form:
*The names of most sugars have end "-ose"
This record includes not only the glucose, but seven isomeric sugars — allose, altrose, mannose, gulose, idose, galactose, talose, characterized by a spatial arrangement of OH-groups and hydrogen atoms at different carbon atoms.
Taking into consideration the arrangement of groups the formula of glucose correct is portray.
Glucose (and any other of the seven isomeric sugars) can exist as two isomers whose molecules are mirror images of each other.
The presence of glucose in any solution can be checked using a soluble copper salt:
In alkaline medium copper salt (II-valent) form with glucose brightly colored complexes (Pic.1 left). When heated, these complexes are destroyed: glucose recovers copper to yellow hydroxide copper (I)-valent) Zion, which is converted into red oxide of Cu2O (Pic. 2 and 3 on the left).
Fructose (fruit sugar) isomery for glucose, but unlike to it relates to caespitum — compounds containing a ketone carbonyl groups
In the alkaline solution these molecules capable of isomerized into glucose, therefore, aqueous solutions of fructose restore copper hydroxide (II-valent) and silver oxide Ag2O (reaction "silver mirror").
Fructose is the sweetest of sugars. It is contained in honey (about 40%), the nectar of the flowers and in the juice of the some plants.
Sucrose (beet or cane sugar)12H22O11 belongs to disaccharides and formed of connected residuals of A-glucose and B-fructose. However, sucrose unlike monosaccharides (A-glucose and B-fructose) does not recover the silver oxide and copper hydroxide (2-valent). In the acidic solution the sucrose hydrolyzed and decomposed by water into glucose and fructose. Here is a simple example: sweet tea seems to be even more sweet if you put in it a slice of lemon, although, of course, and sour. This is due to the presence of citric acid, which accelerates the decomposition of sucrose to glucose and fructose.
If the sucrose solution was mixed with a solution of copper sulfate and add the lye, it will receive bright blue copper saccharate is a substance in which the metal atoms associated with hydroxyl groups of the carbohydrate.
Molecules of one isomers of sucrose — maltose (malt sugar) are composed of two glucose residues. This disaccharide is formed by enzymatic hydrolysis of starch.
.In the milk of many mammals contains another disaccharide, it is saccharose isomer, — lactose (lactose). The intensity of the sweet taste the lactose significantly (three times) inferior then sucrose.
Let's get the milk sugar. This is a sugar found in cow's milk (about 4.5 %) and in woman milk (about 6.5%). Therefore, if the small child gets not women's milk but cow's, the milk must be enriched with milk sugar.
For getting milk sugar, we need the whey – turbid liquid, which is obtained by separation from milk protein and fat with the action of a special enzyme (rennet enzyme). Whey contains small amount of protein, and almost all lactose and mineral salts.
So, in the cup, for example of porcelain, boil on very low heat 400 ml of whey. At this time (the process of boiling) will precipitate from whey remaining protein. After filtration of the protein will continue the boiling till the moment of crystallization of milk sugar. When complete evaporation of the liquid, allow the crystals to cool. Then you will need to separate the milk sugar.
If you want to get a more pure milk sugar, then you need to re-dissolve already received sugar in hot water and repeat the evaporation.
Caramel
If sugar to try to heat, for example, in the cup is higher than the temperature (190 °C), you will notice that the sugar will gradually begin to lose water and break down into component parts. This component is caramel. All you've seen and tasted caramel - you know how she looks is a very viscous yellowish mass, which quickly solidifies when cooled. In the process of formation of caramel the part of the sucrose molecules split into components already known to us - glucose and fructose. And they, in turn, lose water and also break down:The other part of the molecules, which does not decompose into glucose and fructose, enter to the condensation reaction, during which the formation of colored products (caramel 36N50O25 has a bright brown color). Sometimes to get the color effects these substances are added to sugar.
Properties of acids
Getting acids
Íåìåòàëëû
Complex substances. Water hardness
Protein. Properties of proteins
Solid grease.liquid grease. Grease properties
Alcohols. Properties of alcohols
Entertaining experiments. Experiments at home